 Reports on the ADI Team Congress 2011
Reports on the ADI Team Congress 2011
Thursday 14 - Friday 15 April
Manchester Central Convention Complex
Petersfield, Manchester M2 3GX

HTM0105 and implant dentistry
Speakers: Helen Batty, Amy Miller, Helen Frost
Reviewed by Helen McVicker
 Helen Batty
Helen Batty
 Amy Miller
Amy Miller Helen Frost
Helen FrostPreventing the transmission of disease that results from the use of reusable dental instruments, contaminated by blood and secretions, is one of the most critical steps of infection control in our dental practice. In addition to some potentially fatal body-fluid borne pathogens such as HIV and Hepatitis B, the emergence of variant Creutzfeldt Jacob disease has heightened awareness as to the importance of implementing rigorous infection control procedures.
To respond to this heightened state of awareness, over the last decade we have seen dramatic strides in infection control to meet the serious challenges presented by these emerging infections and the ever-broadening spectrum of patients with impaired resistance. One of the most important changes is the emergence of HTM01-05.
This document has very sensible and well laid out objectives, we can easily see the benefits to our practice and our patients.
These are: effective risk reduction, continuous improvement in cross infection protocols, to be-able-to demonstrate consistent processes and outcomes, to make sure that all the equipment and instruments are as clean as possible, and to demonstrate compliance with essential requirements of Medical Devices Regulations 2002 and the Care Quality Commission.
HTM01 05 has two levels of compliance:
- Essential Practice
- Best Practice
Essential practice:
Regardless of the technology used, the cleaned instruments, prior to sterilisation, should be free of visible contaminants when inspected. Instruments should be reprocessed using a validated decontamination cycle including: cleaning / washing (in terms of manual cleaning, this includes having a written protocol); a validated steam steriliser, and at the end of the reprocessing cycle they should be in a sterilised state.
Furthermore, the storage of reprocessed surgical instruments should be in such a way as to ensure restraint of microbiological recolonisation, including controls on the storage times (60 days for a vacuum type autoclave).
You also need to complete an audit every 3 months, and have a plan in place to demonstrate how you are improving and trying to achieve best practice.
Summary of Essential Practice:
![]()
Best Practice:
This is the gold standard that we should all be trying to achieve. It differs from essential practice in that:
- If possible, no more hand cleaning – all cleaning should be done by a validated washer disinfector. Only in very specific circumstances can instruments be disinfected by hand or other methods – this may include the manufacturers specifying that the instrument must be hand cleaned.
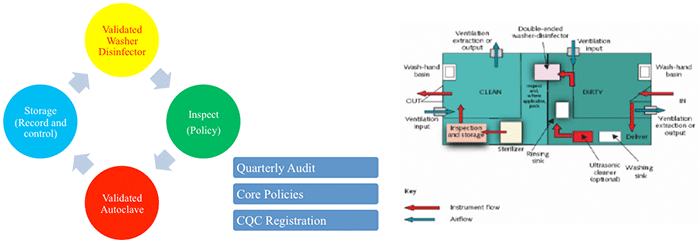
- Instruments have to be stored in a room separate to the treatment areas. And more importantly the whole disinfection process needs to be carried out in separate areas.
- The decontamination facilities should be clearly separate from clinical treatment area. This implies the use of a separate room or rooms for the accommodation of clean (output) and dirty (input) work. In these facilities, the room(s) should be used for this purpose only and access should be restricted to those staff performing decontamination duties.
Summary of Best Practice
Implant manufacturers are doing very little to guide and help us towards best practice, in terms of how to process the instruments that they manufacture. This is unacceptable and frustrating.
Despite the lack of guidance and absence of market leadership, a number of evidence-based recommendations can be suggested:
1: Avoid manual cleaning of instruments
- Ineffective
- Increases risk of needle stick injury
- Not able to validate
- HTM0105 best practice states:
“manual cleaning should only be considered if the manufacturer specifies that the device is not compatible with autonomic processes”
2: Washer disinfector to be used for instruments and burs
- Elimination of splatter
- Avoids needle stick injury
- High temperature destroys vegetative micro-organisms
- Validated
- Not operator sensitive
But.....
- Must conform to BS 2745 and HTM 2030
- Intensive cycle
- In a cassette that allows the jets to be effective or loose in a basket
3: Use single use twist drills
- No method is totally effective at removing debris
- Reduces needle stick injury
- Benefits to operator and patient
- No evidence to inform us if washer-disinfectors damages or blunts the twist drills
4: Pre-soak instruments in enzyme cleaner
- Most effective way to clean
- No evidence to inform us if enzyme cleaner damages the instruments
- Most manufacturers state that ‘ neutral pH safe to use’
An idea best practice cycle would be:
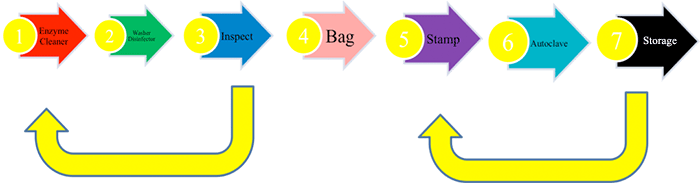
The key to this process in the validation of the cycle, and to relate this to the patient, via their notes. This is done with a sequential number stamp (stage 5) and a date stamp. The number becomes the DECONTAMINATION NUMBER. This logged is against washer-disinfector cycle, autoclave print out, date and duty officer. This number is entered in the patient’s notes.
To conclude, essential practice is fine for now, but we hope that we have gone some way in presenting some guidance of how best practice HTM0105 can be achieved within implant dentistry. We also hope that we have emphasised the concept of getting our instruments as clean as possible, to reduce the cross infection risk for our patients and in a way that reduces the risk to us as dental professionals.
